Atoms, Elements, & Compounds
Activity lab notes to model atoms, elements, & compounds
Questioning is the foundation of all learning.
The first step in rejecting not knowing is to ask, why?
Sweetland
Introduction
An activity to introduce elements, atoms, and compounds with a concrete model using bolts and nuts. With suggestions of a summative activity to create an element fact sheet, book, poster, or leaflet. Some chemical change & ph activities.

Concepts
- Nuts and Bolts can be used to make a model of compounds.
- Compounds are chemically bonded elements.
- An atom is the basic unit of a chemical element.
- Each is roughly 10-8 cm in diameter, consists of a tiny, dense, positively charged nucleus made of neutrons and protons, surrounded by a cloud of negatively charged electrons. Each chemical element consists of atoms that possess a characteristic number of protons. Atoms are held together in molecules by sharing electrons.
- A molecule is a group of atoms bonded together, representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction.
- Element (chemical element) - each element is one of more than one hundred substances that cannot be chemically change to another element or broken down into simpler substances. They are considered primary components of matter. Each element is distinguished by its atomic number, the number of protons in the nuclei of its atoms.
Modeling Atoms, Elements, & Compounds
 Get the following materials
Get the following materials
- 1 long bolt
- 1 short bolt
- 2 hex nuts
- 2 square nuts
Place both kinds of nut atoms and bolt atoms in 4 separate piles on your desk.
Short bolt Hex nut Square nut Long bolt
How many different kinds of atoms do these nuts and bolts represent?
If each kind of atom makes up a different element, How many different elements are represented?
In order to make a compound, at least two atoms have to combine, or be bonded together.
For example, a short bolt atom may be combined (bonded) to a square nut atom.
A sketch representing this combination could be:
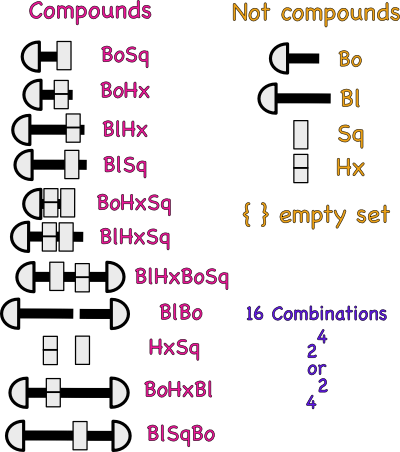
Assuming that Bo is a symbol for a short bolt atom and Sq a symbol for a square nut atom, then the above combination could be described by the following symbol BoSq.
What would the following symbols represent? BoSq2
What does the subscript 2 mean?
Make a sketch representing this combination.
The symbol for a long bolt atom and a hex nut atom are shown below.
Bl Hx
If a long bolt atom and hex nut atom were combined , the what would the shorthand symbol be?
- Use 1 long bolt, 1 short bolt, one square nut and one hex nut to determine how many other different combinations (compounds) can be made.
- Once a combination is made (1) draw it and (2) write its symbol in Data Table #1.
- Then take the combination apart and try again.
- Those already constructed have been entered in the table.
- Darken the heads of long bolts in your sketch.This will serve to distinguish them from short ones.
| Picture of Combination | Shorthand Symbol |
|---|---|
|
BoSq |
|
BlHx |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
How many different compounds did you make?
To be counted as a different compound the combination has to have a different formula.
How do your combinations compare to another teams?
The set of symbols in the table above are also called formulas.
A symbol represents one kind of atom, but, a formula represents a combination of more than one kind of atom. It tells the number and kinds of bolts and nuts (atoms) held (bonded) together.
What is the formula for the combination of two short bolts and two square nuts?
What do the subscripts mean?
Using long bolts and hex nuts, make a compound that
has the formula
Bl2Hx2 and sketch it.
Could it be made in a different way?
If so, sketch it.
What information about a combination is lost by just using the symbol?
Sketch the number of different ways two long bolts can be combined with four square nuts in the table below and write a formula for each. All four nuts have to be used each time.
| Picture of Combination | Formula |
|---|---|
|
|
|
|
|
|
|
|
|
Shorthand symbols could be used to describe how to form a combination (compound). For example, a combination of two long bolts and two hex nuts are threaded together.
This could be described in words
A ( _______________________) is made from
TWO LONG BOLTS ( ______________)
PLUS TWO HEX NUTS ( ______________)
TWO-BOLT-TWO-NUT COMBINATION (______________)
or pictures
or by using symbols
2B l + 2Hx -> Bl2 Hx2
What does the Bl and Hx to the left of the arrow represent?
What does the arrow mean?
What does the formula, Bl2 Hx2 to the right of the arrow mean?
What do the prefix numbers represent on the left side of arrow?
What about the subscript numbers to the right of the arrow?
Create your own interaction with bolts and nuts:
Interaction 1
Draw a Diagram
Write a word description of this interaction.
Now, write a description using symbols.
Interaction 2
Draw a Diagram
Write a word description of this interaction.
Now, write a description using symbols.
How does this relate to the chemisty?
There are millions of different substances in the universe.
However, scientists believe that everything on the earth and the stars are made of various combinations of only about 100 basic substances called elements.
They can be explored in the electronic periodic table of elements supported by the Los Alamos national laboratory.
| Name | Symbol | Name | Symbol | Name | Symbol |
|---|---|---|---|---|---|
| Aluminum | Al | Iodine | I | Oxygen | O |
| Calcium | Ca | Iron | Fe | Potassium | K |
| Carbon | C | Lead | Pb | Silver | Ag |
| Chlorine | CI | Magnesium | Mg | Sodium | Na |
| Copper | Cu | Mercury | Hg | Sulfur | S |
| Helium | He | Nitrogen | N | Zinc | Zn |
| Hydrogen | H |
The penny is made of copper.
What is its symbol?
Many beverage cans are made of aluminum.
What is its symbol?
The oxygen found in air is made of two atoms.
Write its formula.
The nitrogen in air is also made of two atoms.
What would its formula be?
When atoms of elements combine, compounds are formed.
A compound is identified by two things:
- The kinds of atoms in it and
- The number of each kind of atom.
The formula for the compound water is H2O.
What atoms are found in water?
How many of each kind of atom are there?
Complete the table below by adding the missing information.
| Substance | Formula | kind of atom | Number of each Atoms |
|---|---|---|---|
| Water | H2O | H | 2 |
| O | 1 | ||
| Table Salt | NaCl | Na | |
| Cl | |||
| Dry Cleaning Fluid | CC14 | ||
| Sugar | C12H22O11 | ||
| Ammonia | NH3 | ||
| Baking Soda | NaHCO3 | ||
| Rust | FeO | ||
Bolt & nut combinations
Element fact sheet, book, poster, or leaflet
Make an element fact sheet, book, poster, or leaflet (work) to share information about an element. You could collaborate with others with each selecting a different element and creating pages to put in a book.
Suggestions for inclusion:
- Use the name of your atom on the title of your work. Or as a chapter or section in a multi-element book.
- Your name on your work.
- A table of contents for the work. If multiple elements include subcategories for each element.
- Atomic number of your element somewhere in the work.
- Atomic mass of your element somewhere in the work.
- The symbol for your element somewhere in the work.
- A color coded diagram of your element's nucleus showing the particles in the nucleus.
- The number of protons in the nucleus.
- The number of neutrons in the nucleus.
- The electron configuration notation, orbital notation and electron dot notation of your element.
- A short history of your element. Who found it, when was it discovered, etc.
- The appearance of your element in its pure form at room temperature. Is it shiny? yellow? dull? etc. Consider including photos.
- The physical state of your element at room temperature. Is it a solid, liquid or gas?
- How common is your element in nature?
- How could a pure sample of your element be made?
- What are the common uses for your element?
- What are some health effects of your element?
- What are some environmental effects of your element?
- What are the name, formula and uses for one compound containing your element?
- What is the name, formula and uses for another compound containing your element?
- Glossary of key words in your work.
Chemical change activities
Make a cupric sulfate solution and put an iron nail in it for 30 seconds.
Activity Observing a Single Replacement Reaction
Materials
Cupric sulfate crystals, distilled water, beaker or glass container, stirring rod, iron nail, safety goggles and gloves
Prepare the Cupric Sulfate Solution:
- Put on your safety goggles and gloves
- Work in a well-ventilated area
- Measure 20 mL of distilled water into the beaker
- Add approximately 1 gram of cupric sulfate crystals to the water
- Stir until completely dissolved, forming a blue solution.
Procedure
- Carefully place a clean iron nail into the cupric sulfate solution.
- Allow it to sit for 30 seconds.
- Observe
- After 30 seconds, remove the nail
- Observe any changes in color, texture, or deposits on the nail and in the solution.
Explanation
This demonstrates a single replacement reaction where iron displaces copper from the cupric sulfate solution:
CuSO₄ (aq) + Fe (s) → FeSO₄ (aq) + Cu (s)
You'll notice a reddish-brown deposit (copper) forming on the nail, and the solution may change color as iron sulfate forms.
Safety Note: Dispose of chemicals properly and wash your hands after the experiment.
Make an iron sulfate solution and put a magnesium wire into it for 20 seconds.
Activity Observing a Single Replacement Reaction
Materials
10 grams of iron sulfate (FeSO₄), beaker or glass container, distilled water, magnesium wire
Prepare the iron sulfate solution
- Dissolve 2 grams of iron sulfate (FeSO₄) in 20 mL of distilled water and stir until fully dissolved
Procedure
- Put on your safety goggles and gloves
- Work in a well-ventilated area.
- Cut a piece of clean magnesium wire.
- Immerse the wire into the iron sulfate solution for 20 seconds
- Remove the wire after 20 seconds and observe
Observe changes, such as coating formation or color changes, indicative of a chemical reaction.
Dispose of chemicals properly after the experiment.
Acid, base, neutral
Use ph paper to test different liquids for their ph and decide if they are acidic, base, or neutral.
Ph is rated from 0 - 14 with 7 being neutral or 1-12 with 6 being neutral.
Liquids beauty - shampoo, conditioner, face cream, scrubs ...
Drinks - orange juice, tomto juice, milk, different pops, colas ...
Other -